Unraveling Molecular Interactions: Water's Subtle Yet Significant Role
Recent scientific inquiry highlights the profound effect water molecules can have on the structural behavior of chiral catalysts. Chirality, a property describing molecules that are non-superimposable mirror images, is fundamental in fields ranging from pharmaceuticals to materials science. Understanding how these intricate structures interact with their environment, particularly water—a ubiquitous solvent—is therefore of paramount importance. Investigations suggest that even small quantities of water can instigate significant shifts in the preferred configurations of chiral molecules, impacting their function as catalysts and building blocks in synthesis.
Context: Chirality, Catalysis, and the Aqueous Environment
The field of chiral chemistry delves into the spatial arrangement of atoms within molecules and how this arrangement dictates their properties and reactivity. Catalysts, substances that accelerate chemical reactions without being consumed, often rely on specific chiral structures to selectively produce desired molecular outcomes.
Read More: AI Finds Sperm, Skin Cells Made Into Eggs for Fertility Help
Catalyst Design: The efficacy of a chiral catalyst is intricately linked to its three-dimensional shape. Small alterations in this shape can lead to vastly different reaction pathways and product distributions.
Water's Ubiquity: Water is not merely an inert medium; it actively participates in chemical processes. Its polar nature allows it to form hydrogen bonds and influence the molecular conformations of dissolved substances.
Research Focus: Several recent studies have focused on elucidating these water-molecule interactions, particularly concerning chiral structures.
Evidence of Water's Impact
Research published in the Journal of the American Chemical Society has demonstrated that a small number of water molecules can completely alter the preferred structure of prolinol, a molecule commonly employed as a chiral catalyst. This observation underscores the active role water plays in dictating molecular architecture.
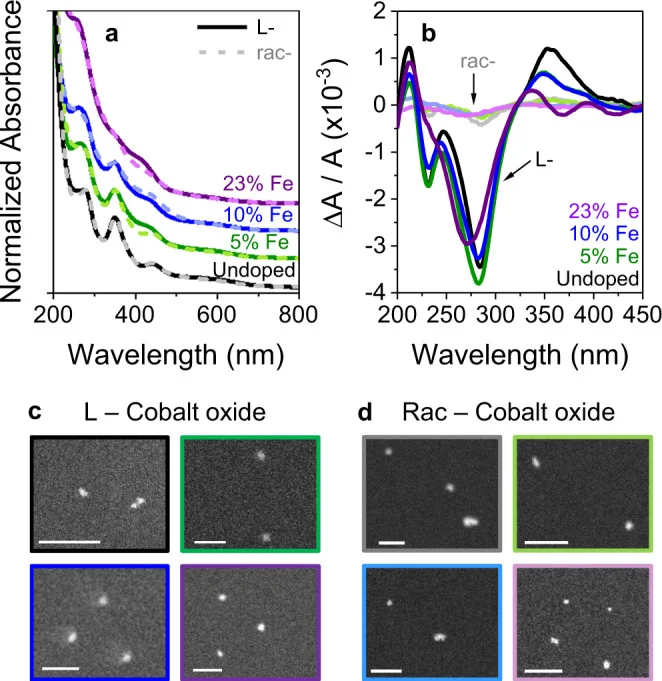
Further research in Nature Communications explored the interplay between chirality, spin properties, and catalysis in the context of water splitting. This study involved chiral electrocatalysts made from cobalt oxide, where spin polarization under a magnetic field influenced oxygen evolution metrics. While this work focused on electrocatalysis, it illustrates how external factors, including spin properties, can be leveraged to manipulate catalytic performance, a principle that may indirectly relate to how solvent interactions could affect catalyst behavior.
Read More: New Ways to Help People Have Babies Using Science
Separately, a study highlighted in PNAS revealed that peptide β-sheets impart their chirality to adjacent water molecules, forming chiral superstructures of water that extend several layers outward. This finding, derived from a combination of spectroscopic techniques and molecular simulations, suggests a reciprocal influence: chiral molecules shape the water around them, creating ordered aqueous environments.
| Study Focus | Key Finding | Publication/Context |
|---|---|---|
| Stepwise hydration of prolinol | A few water molecules can change prolinol's preferred structure. | Journal of the American Chemical Society |
| Spin control in chiral electrocatalysts | Spin-polarized oxygen evolution under a magnetic field was observed. | Nature Communications |
| Chiral superstructures around peptide β-sheets | Peptide β-sheets create ordered chiral water structures extending outward. | PNAS |
Water and Molecular Shape
The findings from the prolinol study suggest a direct and potentially dynamic interaction between water molecules and the catalyst's structure. The addition of just a few water molecules appears to be sufficient to guide the prolinol molecule into a different, stable conformation. This implies that the solvation shell—the layer of solvent molecules surrounding a solute—is not just a passive surrounding but an active participant in determining the solute's spatial arrangement.
Read More: Drinking Coffee and Tea May Lower Risk of Dementia
Chiral Influence on Water Structures
Conversely, the PNAS research demonstrates that chiral molecules can organize water itself. The peptide β-sheets act as templates, inducing a specific, chiral arrangement in the surrounding water molecules. This creates ordered water structures that extend outward from the peptide surface. The question arises: could these water structures then exert a feedback influence on the original chiral molecule's behavior?
Implications for Catalysis and Synthesis
The observation that water can reshape chiral catalysts has significant implications for chemical synthesis.
Reaction Control: Understanding how hydration influences catalyst structure is crucial for controlling reaction outcomes. If water can alter the catalyst's active form, then reaction conditions involving water must be precisely managed.
Catalyst Design: Future catalyst design might need to account for the specific hydrating properties of the intended solvent environment. This could involve designing catalysts that are less susceptible to unwanted structural changes or, conversely, engineering catalysts whose activity is enhanced by specific water-induced conformations.
Biological Relevance: Given water's central role in biological systems, these findings could also offer insights into how biological molecules, many of which are chiral, interact with their aqueous environments to perform their functions.
Expert Analysis
"The idea that a minimal number of solvent molecules can drastically reconfigure a chiral catalyst is quite striking," notes a researcher familiar with hydration studies. "It suggests that the transition states in many catalytic cycles might be more sensitive to solvation than previously appreciated."
Read More: New Way Cells Talk in Tumors Found
Another perspective highlights the dual nature of the interaction: "We're seeing evidence of a molecular dialogue. Not only does the solvent influence the chiral molecule, but the chiral molecule also imposes order on the solvent. The precise nature of this interplay is a fertile ground for future research, particularly concerning its impact on reaction kinetics and stereoselectivity."
Conclusion: A Dynamic Interdependence
The collected research strongly indicates a dynamic interdependence between water molecules and chiral structures. Water is not merely a passive solvent but an active agent capable of reshaping the architecture of chiral catalysts like prolinol. Simultaneously, chiral molecules, such as peptide β-sheets, can impose order on surrounding water, creating structured hydration layers.
These findings underscore the complexity of molecular interactions in solution. For catalysis and chemical synthesis, this means that controlling and understanding the role of water is essential for predictable and efficient chemical transformations. Further investigation into the precise mechanisms of this structural influence and its downstream effects on catalytic activity is warranted.
Sources
Phys.org: "Water molecules actively reshape chiral catalyst structure, research shows"
Published: ~2 minutes ago
Link: https://phys.org/news/2026-02-molecules-reshape-chiral-catalyst.html
Context: General scientific news outlet reporting on recent research.
Nature Communications: "Chiral electrocatalysts eclipse water splitting metrics through spin control"
Published: February 24, 2023
Context: Peer-reviewed scientific journal publishing original research in the natural sciences.
PNAS: "Handy water: Chiral superstructures around peptide β-sheets"
Published: January 8, 2021
Context: Peer-reviewed journal of the National Academy of Sciences, publishing high-impact research across various scientific disciplines.





