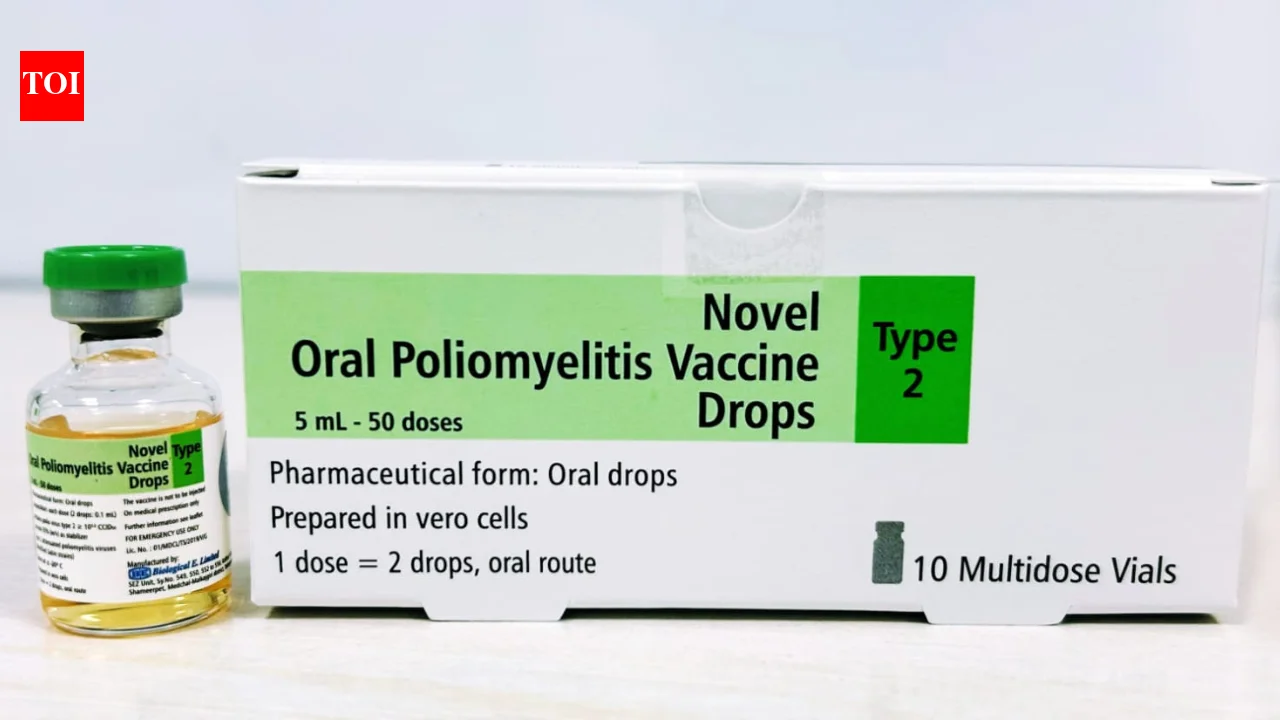
Biological E. Limited (BE), a prominent Indian vaccine manufacturer, has secured Phase II Pre-Qualification (PQ) from the World Health Organization (WHO) for its novel oral polio vaccine type 2 (nOPV2). This advancement signifies a crucial step in BE's capability to provide an end-to-end manufacturing solution for the vaccine, vital in the global effort to combat polio, particularly the circulating vaccine-derived poliovirus type 2 (cVDPV2) strain. The qualification is particularly pertinent for ensuring rapid and flexible vaccine supply to international outbreak control programs.
Context of the Qualification
The recent Phase II PQ for BE's nOPV2 vaccine was granted by the WHO, a significant step beyond the initial Phase I qualification received in June 2024.
Phase I PQ: This initial qualification covered the manufacturing of the drug product using an externally supplied drug substance.
Phase II PQ: The latest qualification extends to both the drug substance and drug product, meaning the entire manufacturing process for nOPV2 now takes place at BE's integrated manufacturing site.
Read More: Know the Early Signs of Colon Cancer
This development addresses the persistent threat posed by cVDPV2 outbreaks, for which nOPV2 is considered a key vaccine. The vaccine's design aims to reduce concerns related to vaccine-associated paralytic polio (VAPP) and possesses improved genetic stability compared to its predecessor, the Sabin poliovirus type 2 (mOPV2) vaccine. This stability is intended to decrease the likelihood of seeding new outbreaks in areas with low immunity.
Evidence Supporting the Milestone
Multiple reports confirm the WHO's pre-qualification status for Biological E.'s nOPV2 vaccine, highlighting various aspects of the achievement.
WHO Pre-Qualification: The World Health Organization has officially granted pre-qualification status to BE's nOPV2 vaccine. This is a rigorous assessment process by the WHO to evaluate the quality, safety, and efficacy of medical products.
Integrated Manufacturing: Phase II PQ specifically covers both drug substance and drug product manufactured at BE's facilities. This confirms BE's capacity for end-to-end nOPV2 production at a single site.
Impact on Outbreak Response: The nOPV2 vaccine is designed to address cVDPV2 outbreaks, with real-world deployments showing a potential to significantly decrease incidence.
Improved Vaccine Characteristics: The vaccine is noted for its improved genetic stability, making it less likely to cause new outbreaks in low-immunity environments compared to older vaccine types.
Regulatory Approvals: Indian regulatory authorities have already approved the vaccine for export purposes.
Read More: Udit Narayan's First Wife Accuses Him and Family of Removing Her Uterus Without Consent
"This progress reflects the critical contributions of PT Bio Farma (Indonesia), PATH, and the Gates Foundation in technical and programmatic support." - Biological E. statement, as cited by IndiaMedToday.
"This expands BE’s WHO-qualified manufacturing scope beyond Phase I, under which the company received PQ in June 2024 for drug product manufacturing using externally supplied drug substance." - Mahima Datla, Managing Director, Biological E.
Enhancing Global Polio Eradication Efforts
The achievement of Phase II PQ by Biological E. has direct implications for global polio eradication strategies.
Continuous and Flexible Supply: The integrated manufacturing capability allows BE to ensure a continuous, flexible, and rapid supply of nOPV2 for international outbreak control. This is crucial because delays in response can put children and communities at risk.
Addressing cVDPV2: The vaccine's specific design targets circulating vaccine-derived poliovirus type 2, a persistent threat in certain regions. nOPV2's improved genetic stability is a key feature in mitigating this risk.
Manufacturer Readiness: With integrated infrastructure and existing export approvals, BE is well-positioned to meet evolving global demand for the vaccine.
Collaborations and Support
The development and qualification of the nOPV2 vaccine involved significant support from various international organizations and foundations.
Read More: AI Finds Sperm, Skin Cells Made Into Eggs for Fertility Help
Bill and Melinda Gates Foundation: Provided a grant to assist BE in meeting the growing global demand for the vaccine.
PT Bio Farma (Indonesia): Contributed to the technical and programmatic aspects of the vaccine's development and qualification.
PATH: Also played a role in providing technical and programmatic support.
Expert Insights
The pre-qualification signifies a major step in BE's journey to contribute to global health security.
"This milestone is a testament to our robust manufacturing capabilities and our commitment to producing high-quality vaccines that can make a significant difference in global health outcomes." - Mahima Datla, Managing Director, Biological E.
The successful qualification means BE's nOPV2 can now be more readily procured and deployed by international organizations for polio control programs. The move underscores India's growing role as a significant contributor to global vaccine supply chains.
Conclusion and Future Implications
Biological E.'s achievement of Phase II WHO Pre-Qualification for its nOPV2 vaccine marks a substantial advancement in its manufacturing capacity and its ability to contribute to global polio eradication. The qualification of both drug substance and drug product at a single, integrated site streamlines production and enhances the potential for rapid deployment.
Read More: Puducherry Leaders Say Elections Are Less Meaningful Without More Power
Manufacturing Excellence: The PQ validates BE's comprehensive manufacturing chain for nOPV2, positioning it as a key supplier for international needs.
Disease Control: The nOPV2 vaccine's improved characteristics offer a more stable and effective tool against cVDPV2 outbreaks.
Global Health Contribution: This development strengthens the global network of vaccine manufacturers capable of responding to public health emergencies, particularly in the fight against polio.
The company is now better equipped to respond to the evolving demands of global polio eradication initiatives, reinforcing the capacity of manufacturers outside of traditional vaccine-producing nations.
Sources Used
IndiaMedToday: https://indiamedtoday.com/biological-e-receives-phase-ii-who-pre-qualification-for-nopv2/
Medicare Pharma Business: https://medicarepharmabusiness.com/biological-e-receives-who-pq-for-novel-oral-polio-vaccine/
The Financial Express: https://www.financialexpress.com/business/healthcare-biological-es-oral-polio-vaccine-gets-who-pre-qualification-3569608/
The Hindu: https://www.thehindu.com/sci-tech/health/who-grants-pre-qualification-status-for-biological-es-novel-oral-polio-vaccine-type-2/article68464617.ece
CIDRAP: https://www.cidrap.umn.edu/polio/who-prequalifies-biological-e-novel-oral-polio-vaccine



:max_bytes(150000):strip_icc()/VWH_EarlySignsofColonCancer-be7dc99d4bfb4a37b2e6bec8d9f4cfd7.png)





